NETL researchers are creating more efficient and environmentally benign electrochemistry technologies that turn carbon dioxide ( CO2) and excess energy back into valuable chemicals and fuels.
One of the challenges associated with power plant economics is excess energy generation. Fossil fuel power plants can’t simply be turned off and on as demand increases or decreases. This picture becomes more complicated when renewables are added to the grid because wind and solar don’t generate a steady supply of power; it’s intermittent as weather conditions vary throughout the day. As a result, over-supply of energy becomes an issue. Storing electricity is not practical because of high costs, low-efficiency and poor reliability of methods for retaining energy that is generated during off peak hours. That’s where electrochemistry comes in.
As its name suggests, “electrochemistry” uses electricity to do chemistry. Electrochemistry research is one way NETL researchers are transforming the reaction science landscape. As NETL researcher Doug Kauffman explained, “we’re basically moving electrons around to make chemistry happen.”
Kauffman said, “If you think about a battery, the positive and negative terminals shuttle electrons around to power devices. If you put two wires on a battery and hook them up to a light bulb, the electrons travel through the bulb and produce light. Electrochemistry uses the same principle, except we’re using those electrons to power chemical reactions instead of lighting a bulb.”
Electrochemistry offers some important benefits. Whereas traditional reaction processes often require high temperatures and pressures, electrochemistry can be conducted at room temperature and in a water-based environment, making it more efficient and environmentally benign. NETL researchers are taking this clean, efficient approach one step further and developing technologies designed to use electricity that would otherwise be wasted to turn CO2 emissions into value-added products – emissions that would otherwise be sent into the atmosphere.
“Electrochemistry can be used to do all sorts of reactions,” Kauffman said. “It can be used to convert carbon dioxide from fossil fuel power generation into other products. CO2 can be dissolved in a liquid, and electricity is applied through electrodes to produce chemicals like carbon monoxide, hydrocarbons, and alcohols. NETL is designing the catalysts needed to create those products.”
NETL researchers use a variety of cutting-edge experimental and computational techniques to design catalysts from the ground up and blaze new trails in CO2 conversion. “We’re focusing on CO2 and the products we want to make. For example, how do we design a material that will turn CO2 into useful chemicals, fuels, or even polymers? We ultimately want to predict precise catalyst recipes so that if we have so many atoms of element A and so many atoms of element B we can selectively and efficiently turn CO2 into a specific product,” Kauffman said.
Using NETL’s world-class facilities and specialized equipment, researchers are designing catalysts for specific chemical reactions. On a microscopic level, materials contain a mixture of shapes and sizes and it’s difficult to tell which particle is doing what. Working with nanocatalysts, NETL researchers are able to better understand and control the chemistry, thereby designing catalysts for optimal energy input, reaction rates, efficiency, selectivity and stability.
The goal is to design a material that will enable reactions at the highest rate and with the least amount of energy. The research team can calculate how efficient, and how selective a catalyst will be.
“Once we find a good material, we’ll put in a prototype bench-scale reactor so we can estimate what the catalyst will look like in a more scalable reactor architecture,” Kauffman said. “This provides a clearer picture of how the material will work in industry-relevant conditions.”
One recent success is a patented gold nanocatalyst that efficiently changes CO2 into fuel. Electrochemical conversion of CO2 typically requires a high amount of energy, but the NETL catalyst makes the process more efficient. By using renewable energy sources to recycle waste CO2 without generating new CO2 emissions, the NETL process results in a net reduction in CO2 gas, making it a carbon-negative energy cycle. If implemented on a commercial scale, NETL’s process has the potential to reduce atmospheric CO2 emissions, aiding efforts to fight climate change.
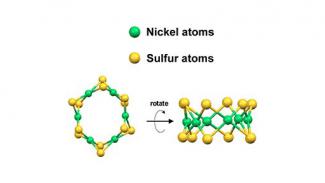
In another breakthrough, researchers developed a nickel-based nanocatalyst that is much cheaper and more efficient than traditional platinum catalysts used for electrochemical systems.
The new catalyst’s atoms are arranged in a tiara-like ring that provides uniform atomic structure. The nickel-based catalyst is extremely stable and overcomes a serious cost-barrier associated with precious metals, like platinum, for large-scale deployment of electrochemical systems. Using inexpensive materials, like nickel, may accelerate the installation of large-scale CO2 conversion systems for producing carbon-friendly, environmentally responsible fuels and chemicals.